A groundbreaking study suggests how a single gene variant shapes the immune landscape of the brain and blood, unlocking new opportunities for early detection and intervention in Alzheimer’s, Parkinson’s, and other neurodogenic disorders.
Study: Apoe of4 carriers share immune-related protective changes in neurodigenerative diseasesImage Credit: Andrii Vodolazhskyi / Shutterstock
In a recent study published in the journal Nature therapyResearchers identified a protected immune signature associated with apolipoprotein E (4 version (4 versionApoe ε4) Ceremonue fluid (CSF), beyond the brain and plasma, regardless of the presence of neurodynative disease.
Apoe ε4 The gene is the most important genetic risk factor for the late starting Alzheimer’s disease (AD). However, explains the growing evidence Apoe ε4 The train can play a role in other neurodigenerative diseases. Apoe ε4 Beginning is linked to a low age and high risk of Parkinson’s disease (PD), amiotrophic lateral sclerosis (ALS), and Frontotemporal dementia (FTD). Apoe ε4 PD dementia (PDD) is also associated with poor cognizance and rapid cognitive decline in the PD, increasing the risk.
Notwithstanding Apoe ε4 The harmful effects, very rarely known about its biological system and what and how it turns into neurodynative diseases. The paper frames it within the evolutionary concept of “antigonistic peliotropy”, suggests that the supporters of genes can be beneficial against infectious diseases in the youth but become harmful with age. First, the authors told that Apoe ε4 The carrier shared a supporter inflammatory protective signature in the CSF regardless of mild cognitive loss and cognitive position in AD. Does this apply to other neurodogenative diseases, which remain unknown.
Study and conclusions
Discovering systemic protective changes in the current study Apoe ε4 Carriers with neurodygenetrable diseases. First of all, the Global Neurodynamon Proteomics Consortium (GNPC) dataset was used for CSF Proteom Profileing of individuals with Somskain Protomic Data, which reflects the clinical inequality of the real world, which reflects the clinical inequality of the real world. Using mutual information, 229 CSF connected to protein Apoe ε4 Eminent controls were identified.
Classification and regression trees (cart) models have shown that these 229 proteins can differentiate Apoe ε4 Carrier from non-guardian in PD and AD. A functional promotion analysis of CSF Apoe ε4 Protein indicated significant enrichment for apoptosis, viral processes, protein folding and phosphorilage, RNA/DNA processes, cellular processes and rhythm.
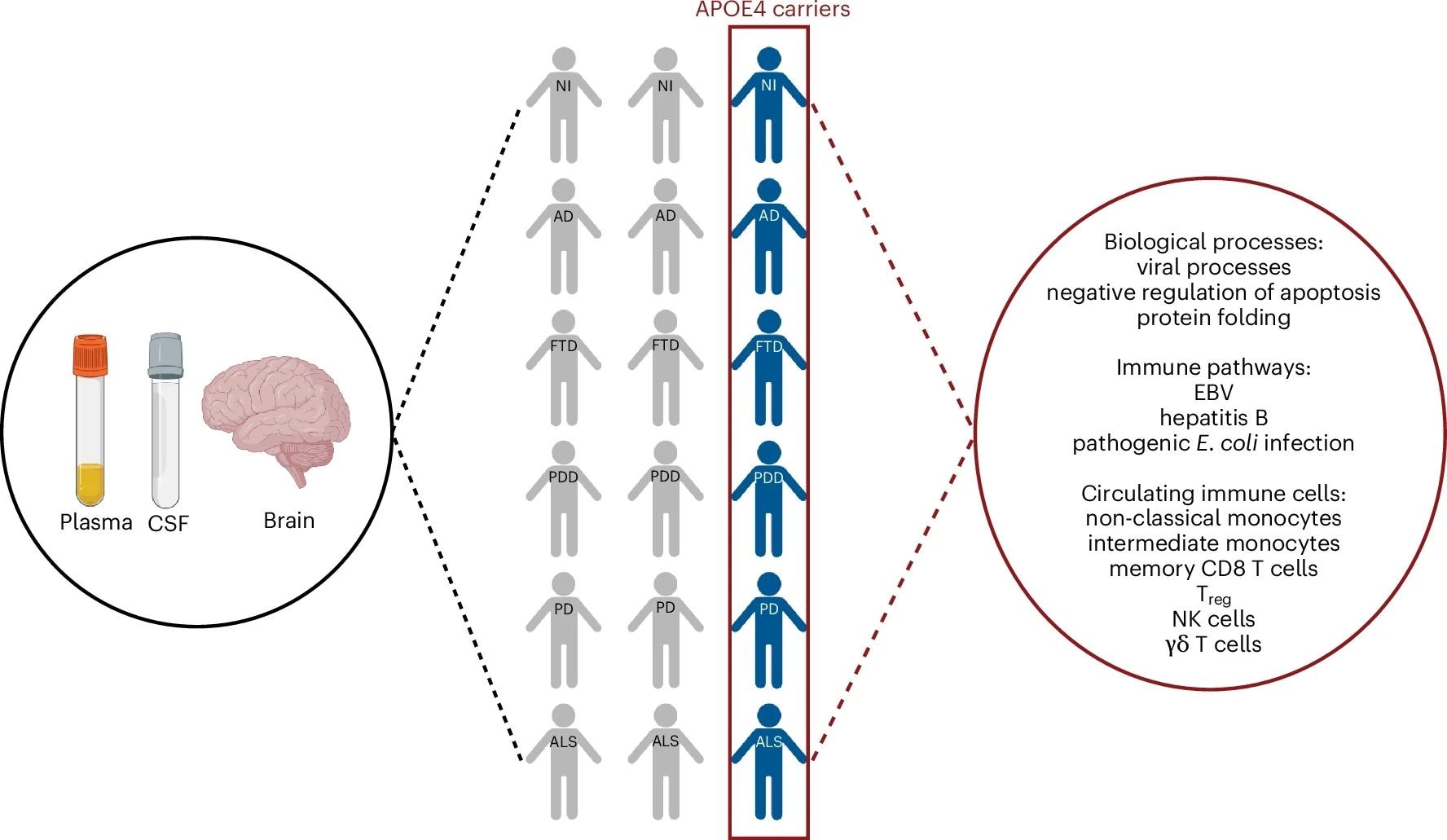 Apoe of4 carriers in various neurodigenerative diseases reflect a common systemic protective change of pro-inflammatory immune deficiency. Made with data Biorender.com,
Apoe of4 carriers in various neurodigenerative diseases reflect a common systemic protective change of pro-inflammatory immune deficiency. Made with data Biorender.com,
Further analysis for immune-specific processes appeared Apoe ε4 Promotion in various transition-related routes including herpes, influenza-e, hepatitis, measles and Epstein-bar virus (EBV). Important promotion for B-cell, T-cell and inflammatory signaling cascade was also seen. Next, immune cell sub -addition analysis revealed the most Apoe ε4 Enrichment in intermediate and non-classical monocytes between congenital immune cells.
In adaptive immune cells, a memory cluster of differentialization 8 (CD8) T cellsRegulatory T (TREG) cells, and memory CD4 T cells were the most rich. In addition, Or T cells and natural killers (NK) cells shown Apoe ε4 Promotion In the liver, a cell-type-specific promotion analysis revealed the most Apoe ε4 Promotion in kufar cells and hepatocytes.
Next, researchers investigated whether Apoe ε4 CSF protrome changes were reflected in plasma and used GNPC dataset for Plasma Protiom Profiling of AD, PDD, FTD, PD, ALS and non-bigure controls. Connected with thirty -eight plasma proteins APO Genotypes were identified in non-beggar controls. Cart modeling showed that these can firmly differentiate between 58 proteins Apoe ε4 Carriers and non-guardians in neurodogenative diseases, and this signature was found to suit various sexes and racial groups.
Apoe ε4 Plasma processes showed significant enrichment in biological processes including protein processes, cellular processes, viral processes, DNA/RNA processes and apoptosis. Viral processes were most importantly rich in both plasma and CSF. It was supported by the same enriches in infection and immune routes including hepatitis and EBV. There were enriched for intermediate and non-classical monocytes and basophils Apoe ε4 Protein between congenital immune cells.
NK cells, γ the t cells, memory CD8T cells, and naive CD8T cells were enriched Apoe ε4 Protein between adaptive immune cells. In the liver, T cells and couples cells were mainly enriched, while hepatocytes showed very little enrichment. These figures indicate that the genotype-specific protective changes found in the CSF were also reflected in the plasma. Apoe ε4 Carrier and non-guard.
In addition, the team discovered whether peripheral protective changes were in the mind of the brain. Apoe ε4 Carrier and non-guard. To end this, they used the protective data of the Dorsolantal Prefrontal Cortex (DLPFC) from individuals with AD, FTD, PD, PDD, ALS, and non-agitated persons, which are part of the faster partnership partnership for AD (AMP-AD). Using mutual information, 248 Apoe ε4 Protein was identified in DLPFC.
Functional enrichment analysis has shown that the three main biological processes (viral processes, apoptosis and protein folded) identified in plasma and CSF were also greatly enriched in DLPFC Apoe ε4 Carriers in neurodygenetrable diseases. Four of the most important rich immune routes in Plasma and CSF were also identified. Apoe ε4 Carrier disease-independent: Hepatitis B, EBV, Escherichia coli Infection, and viral carcinogenesis. Significantly, the study found that this brain signatures were independent of hallmark neurodogenorative pathology, including amyloid-β, tau, tDP-43, and α-synuclein, reinforcing it Apoe ε4 Effect is a fundamental vulnerability rather than direct results of disease-specific protein aggregation.
An unexpected discovery challenges a major biomarker
In a notable and “unexpected discovery”, the study showed that plasma neurophylament light (NEFL) levels, a widely used are used Biomarker Neurodizonation, continuously low Apoe ε4 Carrier. The author focuses on this contradictory few pre-researches and “raises important questions about the reliability of naphl as a stand-alone biomarker,” its level may be suggested. Apoe ε4Metabolic factor or withdrawal beyond blood-brain barrier.
Finally, a correlation was done to examine the relationship between network analysis Apoe ε4 Protein and clinical, demographic and lifestyle factor in PDD, PD, AD, and Non-Bigara Apoe ε4 Carrier. He identified disease-specific relations with APOE in neurodygenetic diseases. Apoe ad was associated with race and sex, to relax diabetes, age, hypertension, and body mass index in non-biguration control, and chronic obstructive pulmonary disease and heart rate in PDD. The author, however, warns that because the study is cross-sectional, they cannot “cause” reasons, “because these clinical factor can be the result of neurodogenative disease rather than a cause.
conclusion
Conclusions revealed that Apoe ε4 Carriers share a unique protective signature in plasma, CSF, and brain regardless of neurodygenetic disease. This signature was associated with enrichment to transmit immune cells and pro -inflammatory immune deficiency. Authors propose a specific mechanism for this, suggesting that hypercarious peripheral immune cells can interact and interrupt with blood-brain barrier (BBB), NeuroinflementHowever, proteins within this signature were specificly correlated with clinical, demographic and lifestyle factors.
this shows that Apoe ε4 A systemic biological vulnerability accepts which is necessary but inadequate for neurodizonation, underlining the requirement of an account for gene-environmental interactions. The author also accepts boundaries, including all clinical diagnosis and “absence of valid biomarkers” to confirm “the absence of direct measures of regular inflammatory markers such as C-Reactive Protein”, which must be addressed in future studies.
Overall, the results start again Apoe ε4 As an AD-specific risk gene provides a foundation for early intervention strategies and accurate biomarker development in neurodygenetic diseases, as a playotropic immune redemption. Calling for an ideological change in the study field, only from identifying genetic risk Loki “leads to the functional characterization of the established variants,” and in these complex interactions moves from installing a roadmap for future research.